Iron mapping
Iron is the most abundant trace element in the human brain and it is essential to biochemical processes as oxygen transportation, myelin production, and neurotransmitter synthesis. However, it can become toxic when available in excessive amounts. Abnormal high iron concentrations have been linked to neurodegenerative and inflammatory processes occurring in prevalent neurological disorders such as Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. A prerequisite for investigating the role of iron in the etiology and disease progression is the precise assessment of its concentration.
Techniques for iron mapping
Several techniques have been proposed for the assessment of iron concentration in the brain including R2 and R2* Relaxometry (R2*=1/T2*), phase imaging, quantitative susceptibility mapping (QSM), and direct saturation imaging (DSI).
|
|
|
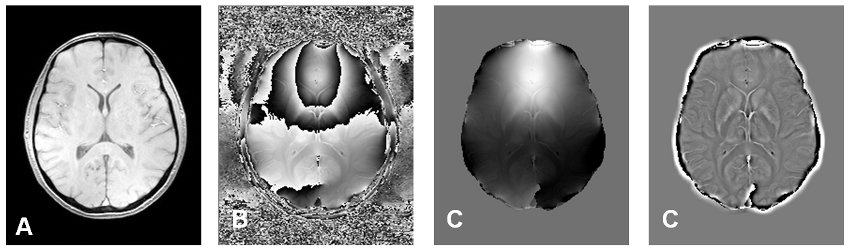
Figure: Paramagnetic iron is not only changing relaxation times of tissue (A), but also the phase of a gradient echo (B). To obtain maps representing susceptibility induced field shifts, typical 2-pi periodic phase wraps have to be unwrapped (C) and filtered (D) |
Validation of iron mapping methods
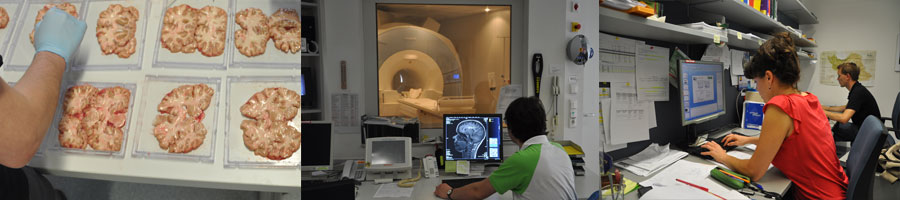
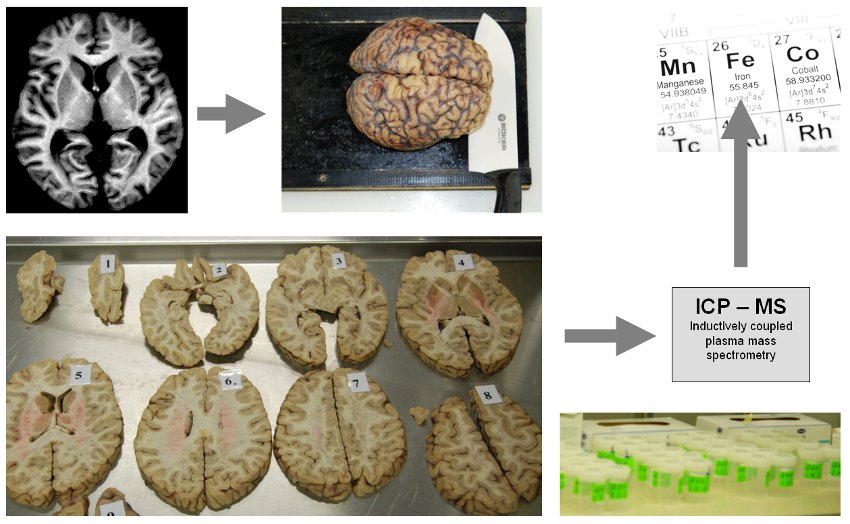
MRI is the only available technique which can assess indirect effects of iron in-vivo and therefore enables longitudinal investigations in the human brain. Because it was unclear so far which MR technique provides valid and sensitive measures for iron, postmortem studies of the human brain were performed for a chemical validation. Iron concentrations were determined in a variety of anatomical brain regions using various MRI techniques and, additionally, using inductively coupled plasma mass spectroscopy.
|
|
| Figure: Postmortem studies including in situ MRI directly after death and chemical analysis of regional brain iron concentrations enable a direct validation. In our work, the formalin-fixed brains were cut into 10mm-thick brain slices and tissue specimens were subsequently referred to an inductively coupled plasma mass spectrometer. |
Clinical applications of iron mapping - in-vivo
Based on these measurements a model of the biophysical mechanisms underlying MRI contrast generation in the human brain was developed and subsequently applied in clinical studies. The results showed that iron levels are elevated in the basal ganglia of multiple sclerosis (MS) patients. In patients suffering from amyotrophic lateral sclerosis (ALS) white matter changes in the corticospinal tract were paralleled by increased focal iron deposition. Current work focuses on MS, ALS, Parkinson´s disease, Alzheimer´s disease as well as the cause of iron accumulation in the process of normal aging.
Heritability of brain iron
Heritability heritability of regional brain iron concentrations, assessed by R2* relaxometry at 3 Tesla MRI, were estimated with variance components models in 130 middle-aged to elderly participants of the Austrian Stroke Prevention Family Study. Heritability of R2* iron ranged from 0.46 to 0.82 in basal ganglia and from 0.65 to 0.76 in cortical lobes. Age and BMI explained up to 12% and 9% of the variance of R2* iron, while APOE ε4 carrier status, hypertension, diabetes, hypercholesterolemia, sex and smoking explained 5% or less. The genetic correlation of R2* iron among basal ganglionic nuclei and among cortical lobes ranged from 0.78 to 0.87 and from 0.65 to 0.97, respectively. R2* rates in basal ganglia and cortex were not genetically correlated. Regional brain iron concentrations are mainly driven by genetic factors while environmental factors contribute to a certain extent. Brain iron levels in the basal ganglia and cortex are controlled by distinct sets of genes.
References
**Hofer E, Pirpamer L, Langkammer C, Tinauer C, Seshadri S, Schmidt H, Schmidt R.
Heritability of R2* iron in the basal ganglia and cortex.
Aging (Albany NY). 2022 Aug 9;14(16):6415-6426. doi: 10.18632/aging.204212. Epub 2022 Aug 9. PMID: 35951362; PMCID: PMC9467397.
** Birkl, C; Langkammer, C; Krenn, H; Goessler, W; Ernst, C; Haybaeck, J; Stollberger, R; Fazekas, F; Ropele, S
Iron mapping using the temperature dependency of the magnetic susceptibility.
Magn Reson Med. 2014; 43(5):
** Ropele, S; Wattjes, MP; Langkammer, C; Kilsdonk, ID; de Graaf, WL; Frederiksen, JL; Fuglø, D; Yiannakas, M; Wheeler-Kingshott, CA; Enzinger, C; Rocca, MA; Sprenger, T; Amman, M; Kappos, L; Filippi, M; Rovira, A; Ciccarelli, O; Barkhof, F; Fazekas, F
Multicenter R2* mapping in the healthy brain.
Magn Reson Med. 2013; 65(8)
** Langkammer, C; Liu, T; Khalil, M; Enzinger, C; Jehna, M; Fuchs, S; Fazekas, F; Wang, Y; Ropele, S
Quantitative susceptibility mapping in multiple sclerosis.
Radiology. 2013; 267(2):551-9
** Langkammer, C; Ropele, S; Pirpamer, L; Fazekas, F; Schmidt, R
MRI for Iron Mapping in Alzheimer's Disease.
Neurodegener Dis. 2013; 369(7) PubMed
** Langkammer, C; Krebs, N; Goessler, W; Scheurer, E; Yen, K; Fazekas, F; Ropele, S Susceptibility induced gray-white matter MRI contrast in the human brain.
Neuroimage. 2012; 59(2):1413-1419
** Langkammer, C; Schweser, F; Krebs, N; Deistung, A; Goessler, W; Scheurer, E; Sommer, K; Reishofer, G; Yen, K; Fazekas, F; Ropele, S; Reichenbach, JR Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study.
Neuroimage. 2012;
** Khalil, M; Langkammer, C; Ropele, S; Petrovic, K; Wallner-Blazek, M; Loitfelder, M; Jehna, M; Bachmaier, G; Schmidt, R; Enzinger, C; Fuchs, S; Fazekas, F Determinants of brain iron in multiple sclerosis: a quantitative 3T MRI study.
Neurology. 2011; 77(18):1691-1697
** Khalil, M; Teunissen, C; Langkammer, C Iron and neurodegeneration in multiple sclerosis.
Mult Scler Int. 2011; 2011(11):606807-606807
** Ropele, S; de Graaf, W; Khalil, M; Wattjes, MP; Langkammer, C; Rocca, MA; Rovira, A; Palace, J; Barkhof, F; Filippi, M; Fazekas, F MRI assessment of iron deposition in multiple sclerosis.
J Magn Reson Imaging. 2011; 34(1): 13-21.
** Langkammer, C; Enzinger, C; Quasthoff, S; Grafenauer, P; Soellinger, M; Fazekas, F; Ropele, S Mapping of iron deposition in conjunction with assessment of nerve fiber tract integrity in amyotrophic lateral sclerosis.
J Magn Reson Imaging. 2010; 31(6): 1339-1345.
** Langkammer, C; Krebs, N; Goessler, W; Scheurer, E; Ebner, F; Yen, K; Fazekas, F; Ropele, S Quantitative MR imaging of brain iron: a postmortem validation study.
Radiology. 2010; 257(2):455-462