Preclinical MRI
The overall objective of our collaborative research is to develop novel drug delivery systems based on nanoparticles for the specific transport of Alzheimer disease drugs over the blood brain barrier (BBB). The nanoparticles as drug carriers are prepared based on biodegradable polymers such as human serum albumin (HSA). Alzheimer disease drugs are incorporated into the nanoparticle matrix. The surface of the drug-loaded nanoparticles is modified by a covalent attachment of apolipoproteins for facilitating the transport over the BBB. Due to an established synthesis protocol for unloaded nanoparticle formulations a fast developing process of Alzheimer disease drug loaded nanoparticles is expected. Our partners are developing suited in vitro and in vivo test systems to show the efficiency of these nanoparticle formulations for treatment in Alzheimer disease. This transport will be monitored on single cell basis to specifically demonstrate the cellular transport mechanism. The specific involvement of lipoprotein receptors will be shown by specific inhibitors and knock-in mouse models and the biological activity of transported GSMs will be monitored in in vitro blood brain barrier models and in vivo using transgenic mice resembling the AD pathology.

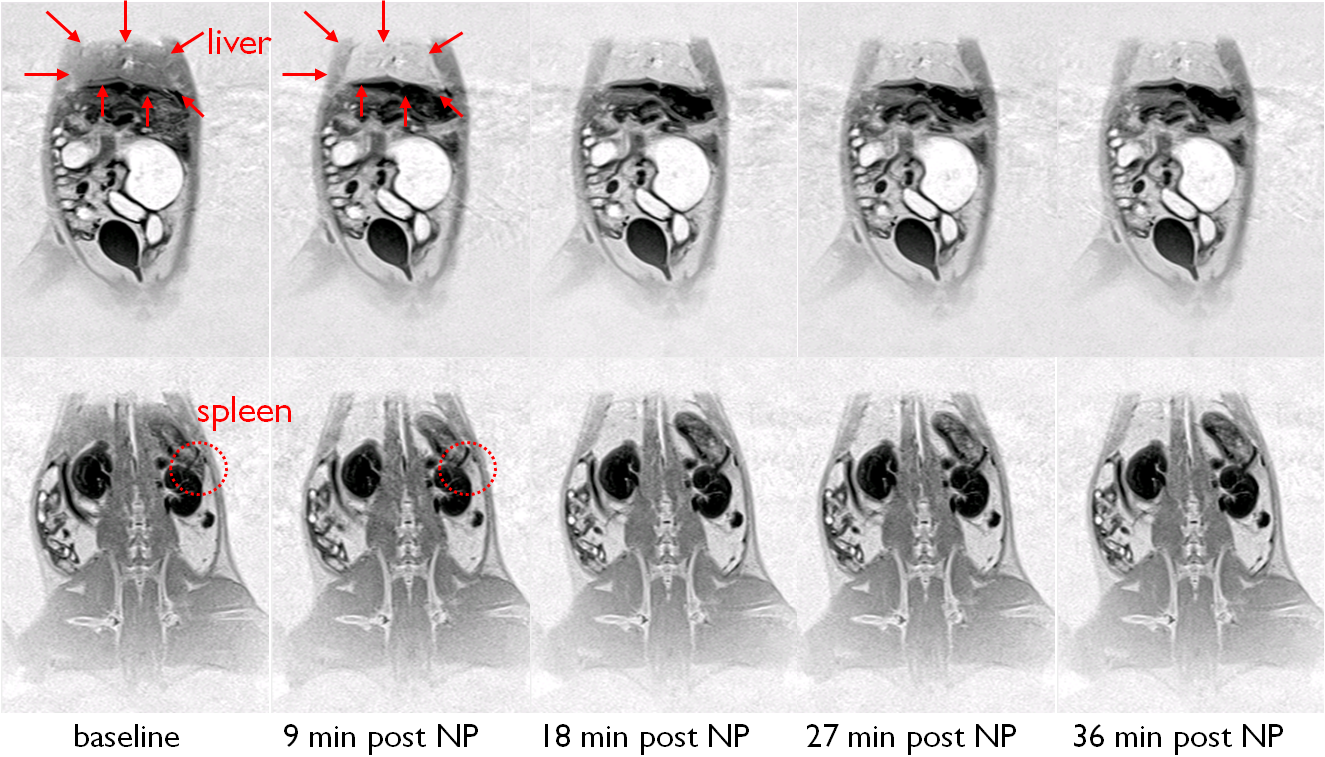
Our goal is the development of a new bioactive formulation of the nanoparticles which allows their tracing by MRI. MRI is a well-suited tool for tracking these nanoparticles because of its non-invasive nature and its high spatial resolution. Nanoparticles themselves do not induce intrinsic MR contrast. To produce a strong contrast they have to be labelled with paramagnetic particles such as Gd-based compounds, ultrasmall paramagnetic iron oxides (USPIOs) or superparamagnetic iron oxides (SPIOs). Paramagnetic material changes the microscopic and macroscopic magnetic susceptibility of the tissue and therefore can be made visible with T1 weighted or susceptibility weighted sequences.
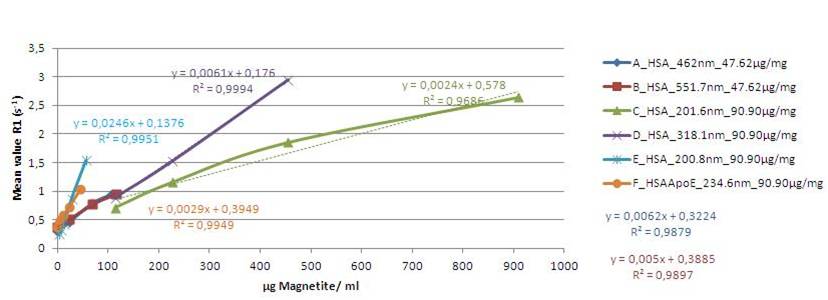
In our current research we are testing and identifying paramagnetic compounds including iron particles that can be used best to track the nanoparticles in mice. Additionally, we are optimizing susceptibility weighted MR sequences and a T1 weighted sequence to maximize sensitivity for the labelled nanoparticles and to improve contrast to noise ratio while preserving spatial resolution. Sensitive tracking of the nanoparticles in transgenic mice using optimized compunds and sequences will allow us to learn more about the pharmacological dynamics of the nanoparticles and their accumulation in the AD brain.